Abstract
INTRODUCTION Pathogenic T cells cause many diseases including most autoimmune diseases and graft-versus-host disease (GVHD). Selectively targeting these pathogenic T cells while sparing the normal T cells and other tissues is a "holy grail" of therapeutics development in modern clinical immunology. So far, pan-immunosuppressive drugs such as corticosteroids are used to treat these patients, with limited efficacy and severe adverse effects. It is also well-established that these pathogenic T cells, whether auto- or alloreactive, proliferate following antigen recognition to cause tissue damage while the other normal T cells remain quiescent. Selectively targeting the proliferating T cells could be an effective strategy to develop new drugs for diseases mediated by the pathogenic T cells.
Antibody-drug conjugates (ADCs) are developed by conjugating a potent toxin onto a monoclonal antibody (mAb) specific for a cancer cell surface antigen. Monomethyl auristatin E (MMAE), a synthetic mitotic toxin, is the payload in several FDA-approved ADCs, and it kills the actively dividing cancer cells by blocking the polymerization of tubulin, rapidly inducing apoptosis. These cancer cells and pathogenic T cells have one feature in common-both of them are actively proliferating, thus, this ADC approach proven successful in cancer treatment could be re-purposed to selectively kill pathogenic T cells for the treatment of T cell-mediated diseases.
CD6 is a cell surface glycoprotein that is expressed at high levels on all T cells except Treg cells, a small portion of B cells and many human NK cells. CD6 is not detectable on other tissue cells, making it a highly specific target candidate for T cells. We have generated CD6 knockout mice and CD6 humanized mice, and developed anti-CD6 mAbs that treat mouse models T cell-mediated diseases. A CD6-targeted ADC (CD6-ADC) thus might be effective for treating T cell-mediated diseases by selectively eliminating the proliferating pathogenic T cells.
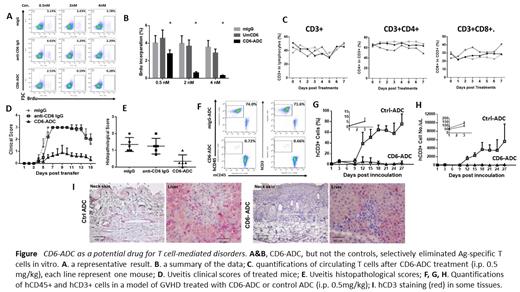
METHODS A CD6-ADC was developed by conjugating a latent form of MMAE onto the high-affinity anti-human CD6 mAb. Its potency of selectively killing pathogenic T cells was evaluated in an antigen-specific T cell recall assay with BrdU-incorporation followed by flow cytometric analyses. To determine the toxicity of the CD6-ADC on normal quiescent T cells, naïve CD6 humanized mice were treated with the CD6-ADC or non-binding control ADC (0.5 mg/kg) by intraperitoneal injection, then numbers of circulating T cells were monitored by flow cytometry daily. To evaluate the efficacy of CD6-ADC in treating T cell-mediated autoimmune diseases, the same dose (0.5 mg/kg) of CD6-ADC, or the parental anti-CD6 mAb, or the control ADC was used to treat a model of autoimmune uveitis, and disease severities were assessed by various ocular imaging techniques including indirect ophthalmoscopy, confocal scanning laser ophthalmoscopy and optical coherence tomography. To examine the potential of the CD6-ADC in treating GVHD, the same dose (0.5mg/kg) of CD6-ADC or control ADC was tested in a model of GVHD induced in NSG mice after adoptive transfer of human PBMC. GVHD severities were assessed by flow cytometric analyses of circulating human T cells and by histopathological analyses of different tissues.
RESULTS: The CD6-ADC selectively killed antigen-specific proliferating T cells in vitro. Treating naive CD6-humanized mice with this CD6-ADC (0.5 mg/kg) did not significantly eliminate normal T cells in vivo. Furthermore, systemic delivery of the same dose (0.5 mg/kg) of CD6-ADC, but not the anti-CD6 mAb alone nor the control IgG effectively reduced retinal inflammation in a preclinical model of autoimmune uveitis. The same dose of CD6-ADC, but not the control ADC, also effectively depleted activated xenogeneic T cells and prevented the development of GVHD in NSG mice after the adoptive transfer of human PBMC.
CONCLUSION: These data indicate that this CD6-ADC holds promise as a new drug for treating diseases in which T cells are integrally involved in the pathogeneses such as GVHD.
Lin: Takeda Pharma: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal